
FDA aims to reduce use of animal products for dental bone grafts
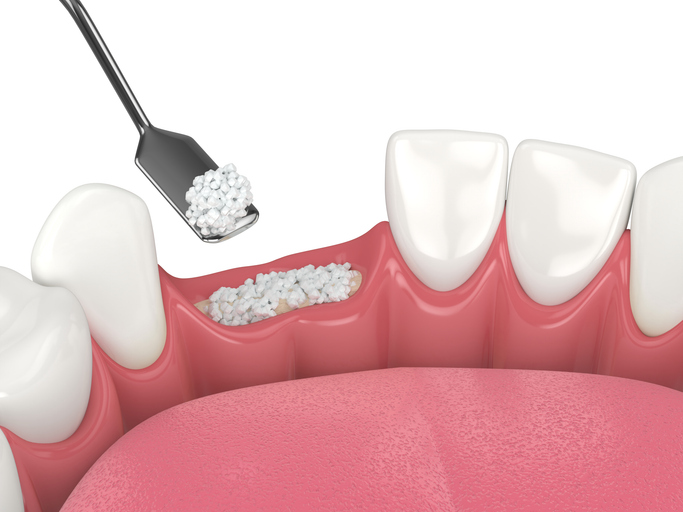
The Food and Drug Administration has released finalized guidance for manufacturers of dental bone grafting material devices. The key aim is to reduce reliance on animal testing by promoting transparency and encouraging alternative testing methods.
The guidance supports the 3Rs principle: replace, reduce and refine animal use in testing. Manufacturers are encouraged to consult with the FDA about using nonanimal alternatives. The new guidance helps companies meet regulatory "special controls" while minimizing animal testing.
Bone grafting is on the rise, driven by regenerative medicine and increased demand for dental implants. Up to 50% of dental implant procedures use bone grafts. Many grafts use xenografts — animal-derived materials — primarily from cows or pigs.
Read more: FDA
The article presented here is intended to inform you about the broader media perspective on dentistry, regardless of its alignment with the ADA's stance. It is important to note that publication of an article does not imply the ADA's endorsement, agreement, or promotion of its content.